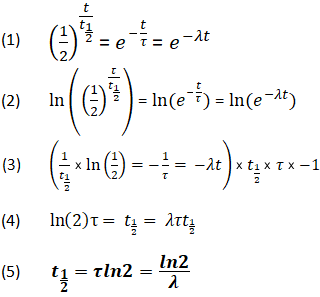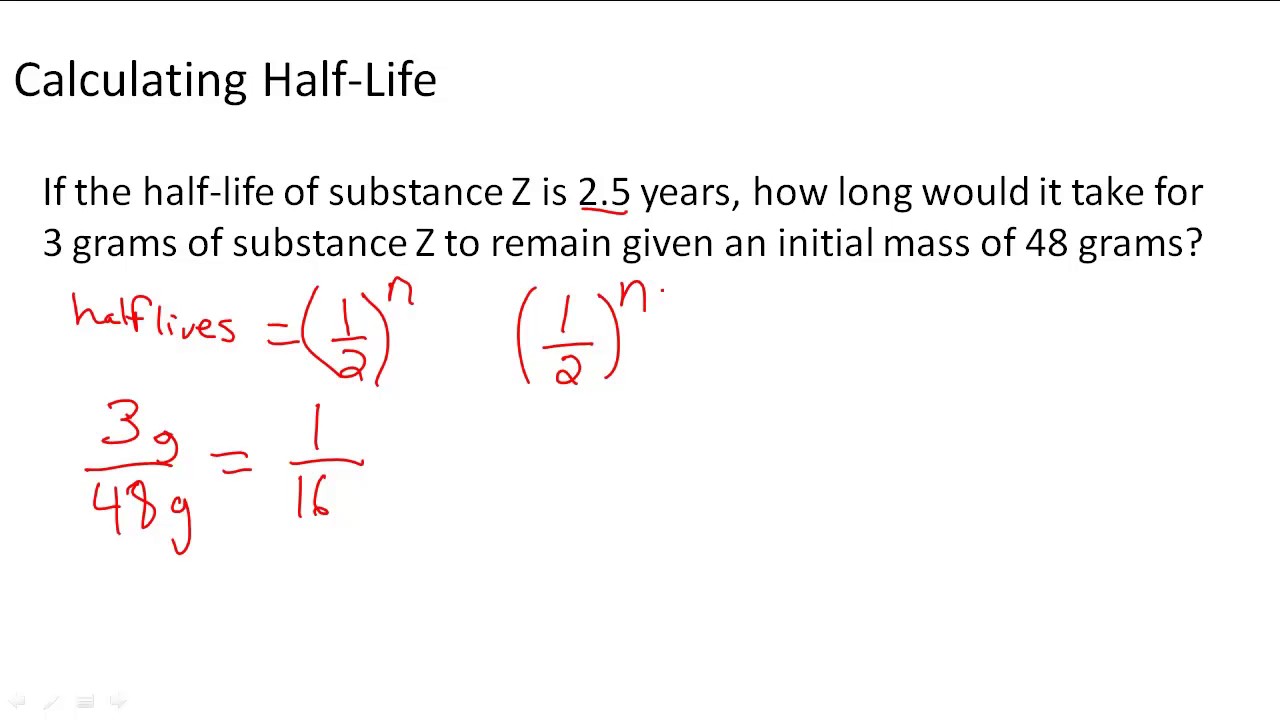half life formula physics
The term is most commonly used in relation to atoms. So suppose a sample has a count rate of 3200 Becquerel Bq at the start what its count rate would be after 8 days would be 116th of.
Half-life is the time required for the amount of something to fall to half its initial value.

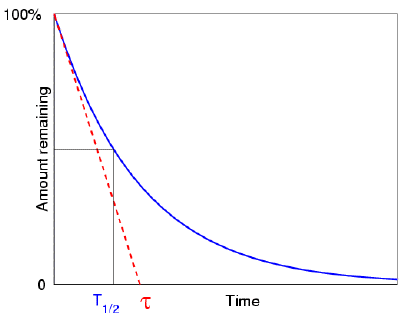
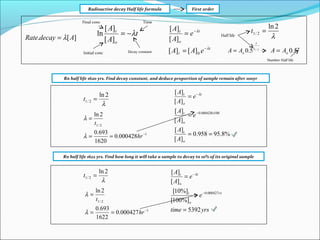
. To see how the number of nuclei declines to half its original value in one half-life let t t 12 in the exponential in the equation N N 0 e λtThis gives N N 0 e λt N 0 e 0693 0500N. N is the number of nuclei left in the sample while T is the number of halvesIn the graph there are a total of 5 half-lives T 12 being the symbol for half-life and nT being the number of half-lives. The term is commonly used in nuclear physics to describe how quickly unstable atoms undergo radioactive decay or how long stable atoms survive.
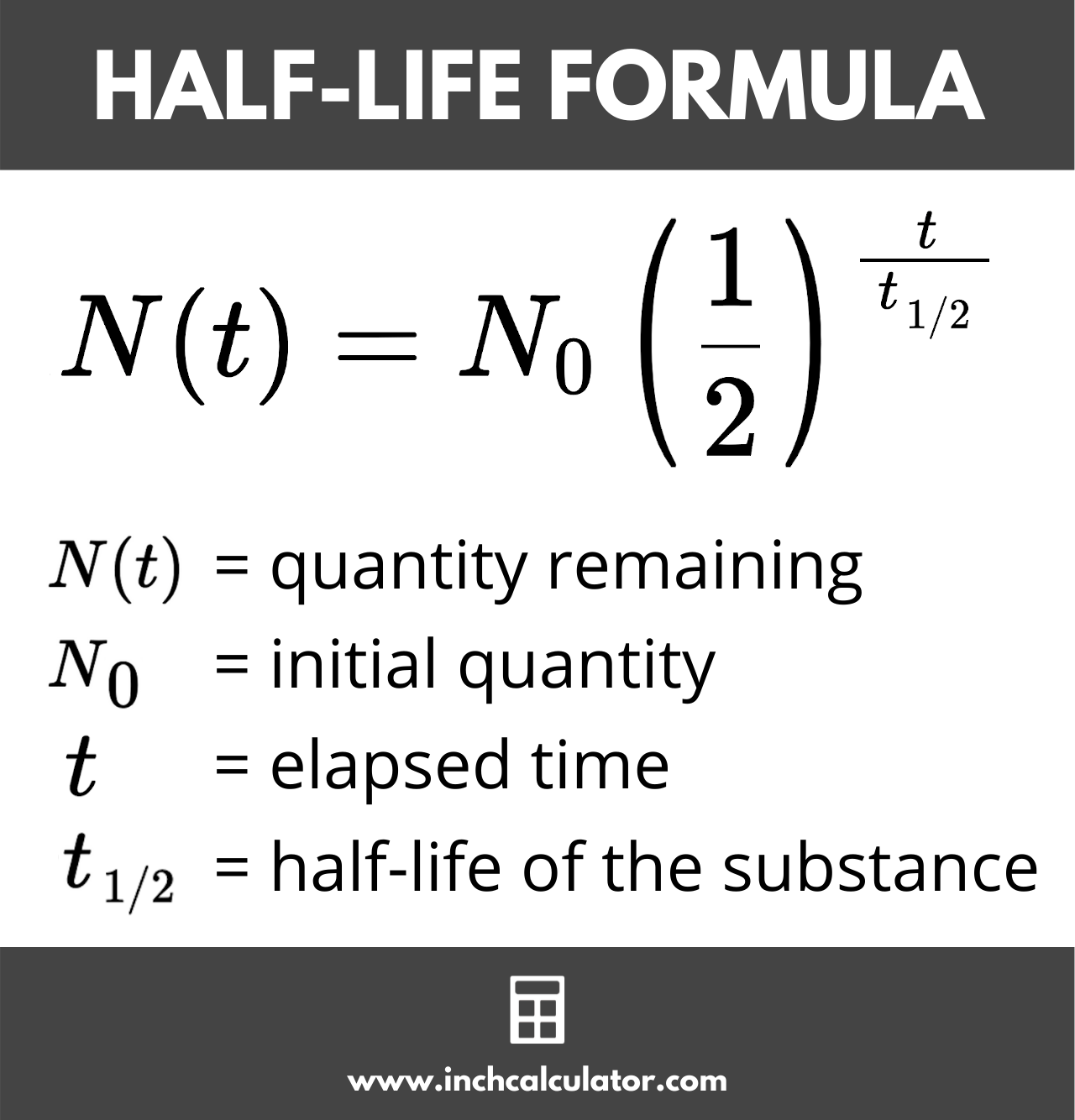
Half-life is defined as the amount of time it takes a given quantity to decrease to half of its initial value. Half-life symbol t12 is the time required for a quantity to reduce to half of its initial value. 9 rows The isotopic distribution of potassium on the Earth is approximately 93 39 K and 7 41 K.
Understand what half-life is and its applications in chemistry and physics. So if the half-life is two days four half-lives is 8 days. Ad Browse Discover Thousands of Science Book Titles for Less.
It is the time requires to decay in half. When half of the radioactive atom undergoes the decay process the time needed for a quantity to reduce to half of its initial value is the half-life. The mathematical representation of Half life is given by Half life.
Half-life formula and unit for first order reaction. T12 2 years. Explore the equation for half-life various half-life examples and worked-out problems.
The half-life formula used to calculate the first-order reaction is t₁₂. The half-life formula is commonly used in nuclear physics where it describes the speed at which an atom undergoes radioactive decay. The half-life of some radioactive elements is very small for example the half-life of.
For example the medical scienc. The term is also used more generally to characterize any type of exponential or rarely non-exponential decay. The symbol for half life is usually τ12.
Definition of the Half-Life. Consider a radioactive substance with a mass of 4kg and a. T 12 0693 λ.
For example the half-life of uranium-238 is 45 10 9 years while the half of radium-226 is 1620 years. This expression confirms that the time it takes for a radioactive sample. The formula for the half-life is obtained by dividing.
T 12 0693 03465 2 years. The value of the half-life is given by. The unit of half-life equation for zero order reaction is second 2.
Since these values are only approximate the total percent abundance of these two isotopes. Half life formula- The time taken for half of reactions to complete or the time at which the concentration of the reactant is reduced to half of its original value is called the half life period. If we now focus on a ratio of one-half we can find the expression for the half life.
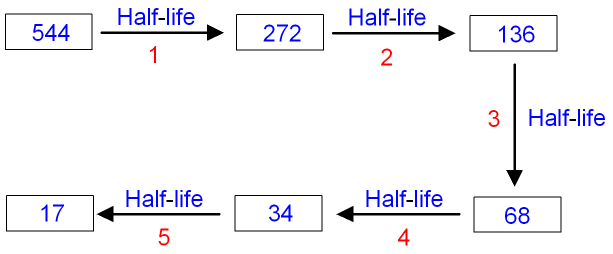
Gcse Physics How Can Half Life Be Used To Calculate The Count Rate Gcse Science
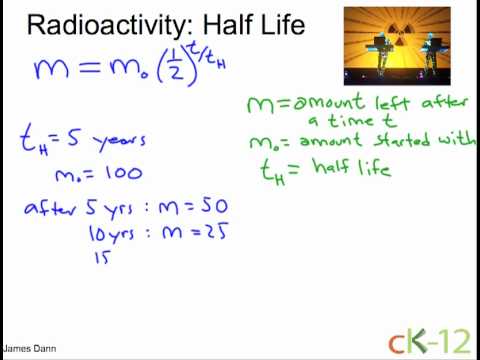
Radioactivity Half Life Youtube
Radioactive Decay Equation Formula Nuclear Power Com

5 Ways To Calculate Half Life Wikihow

Half Life And Carbon Dating Video Nuclei Khan Academy
Advanced Level Physics Calculating Half Life Powerpoint And Lesson Plan

Grade 11 Physics Nov 21 Halflife And Fission

How To Calculate Half Life Of A First Order Reaction Chemistry Study Com
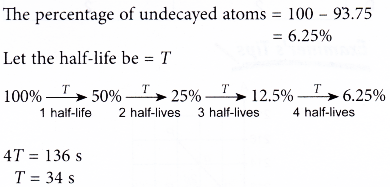
What Is The Half Life Of A Radioactive Element A Plus Topper
Option C Nuclear Physics Radioactive Decay And Half Life
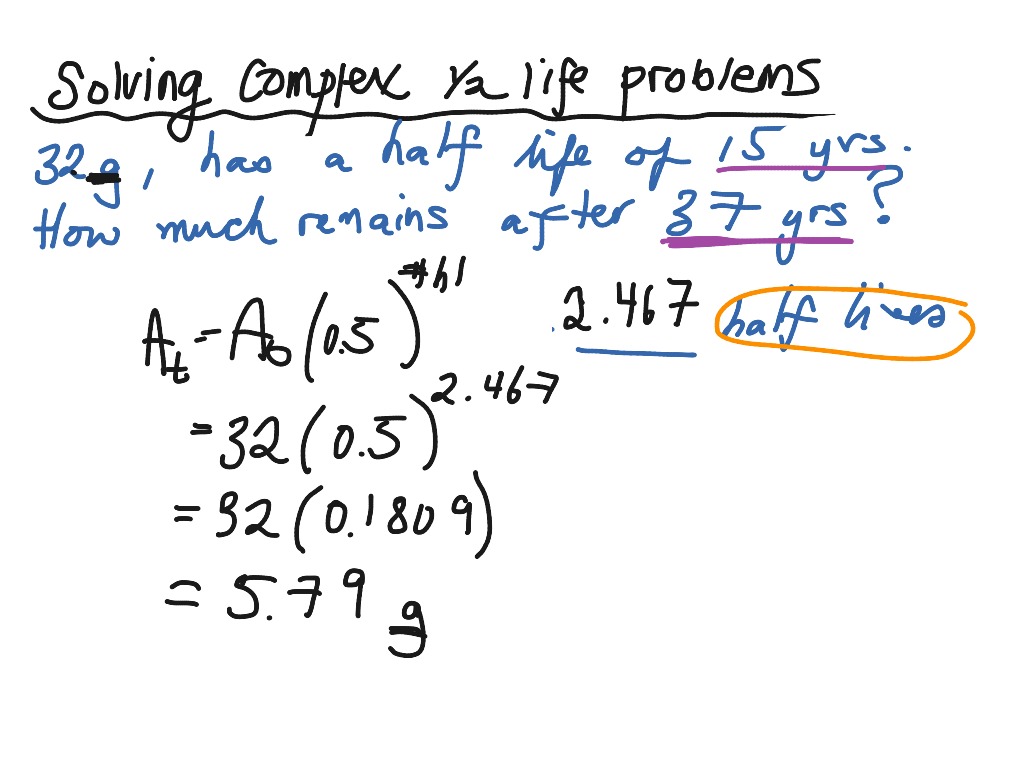
Using The Half Life Formula For More Complex Problems Science Chemistry Nuclear Chemistry Showme

Tips For Half Life Calculations Concept Chemistry Video By Brightstorm

Derivation Of Half Life Youtube